AI-Designed Antibiotics are no longer science fiction — they are here. In a landmark MIT study published in Cell, generative AI created two novel compounds capable of killing drug-resistant gonorrhoea and MRSA in laboratory and animal tests. This achievement not only demonstrates the power of AI in antimicrobial research but also signals a potential turning point for pharmaceutical R&D.
Study Summary (What Happened)
Researchers at MIT announced that AI-Designed Antibiotics have successfully killed gonorrhoea and MRSA, two superbugs identified by the WHO as urgent threats.
- Designed atom-by-atom by generative AI
- The AI system analyzed chemical structures and bacterial response data, then generated novel molecular designs.
- Two approaches were used: one starting with small fragments of 8–19 atoms, and another giving AI free rein from scratch.
- Filtering for drug-like properties
- Models excluded designs too similar to existing antibiotics, compounds predicted to be toxic, or substances resembling non-medicinal chemicals.
- Lab and animal testing
- From 36 million possible compounds, scientists synthesized and tested a handful in lab experiments and in infected mice.
- Two AI-Designed Antibiotics candidates showed promise in killing the superbugs.
- Limitations and challenges
- Out of 80 gonorrhoea candidates, only 2 could be successfully manufactured.
- Both drugs still require 1–2 years of refinement before entering the long path of clinical trials.
- Experts stress that while AI accelerates discovery, testing for safety and efficacy remains long, costly, and uncertain.
- There are also economic hurdles: antibiotics are ideally used sparingly to prevent resistance, making them commercially unattractive.
Prof. James Collins (MIT) called the findings a potential start of a “second golden age in antibiotic discovery.” Other experts hailed the work as a significant step forward, while warning that challenges in manufacturing, regulation, and economics remain.
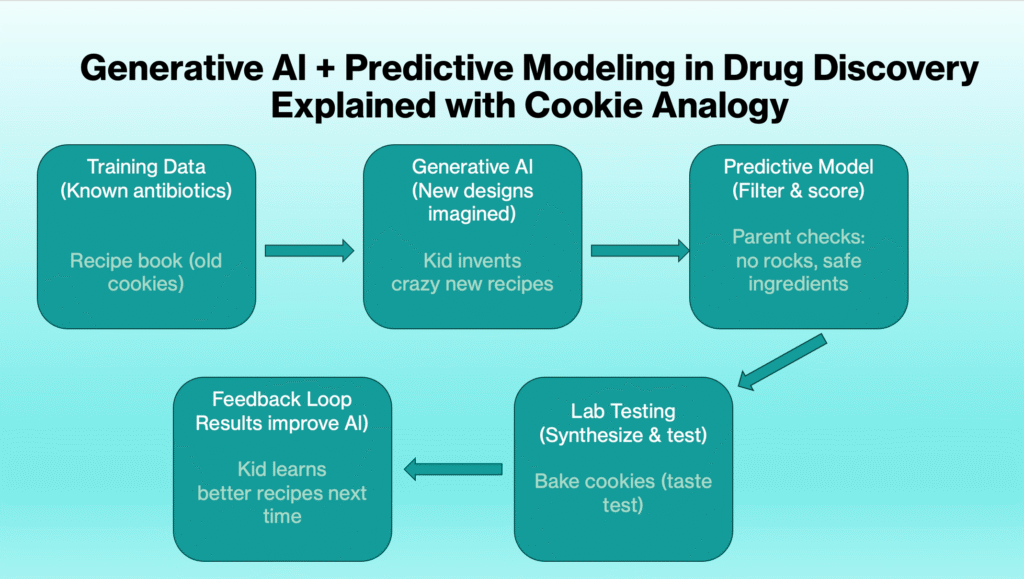
Explaining the Methods (Generative AI + Predictive Modeling)
AI-Designed Antibiotics were created through a combination of generative AI and predictive modeling.
To understand what makes this special, let’s break down the method.
Generative AI
- Think of it as an artist that can imagine brand-new Lego structures.
- Instead of reusing the same old bricks, it dreams up completely new shapes and designs — piece by piece, atom by atom.
Predictive Modeling
- Now imagine a “wise judge” who looks at each Lego design and says:
- “This one will be strong.”
- “This one will fall apart.”
- “This one looks unsafe.”
- Only the strongest designs get picked to actually build in real life.
The Discovery Loop
- Train AI on past antibiotics (what worked, what didn’t).
- AI imagines millions of new designs.
- Predictive model filters out the bad ones.
- Scientists test the top picks in the lab.
- Results feed back to the AI to make it smarter next round.
👉 Explained like you’re 6:
It’s like baking cookies.
- The AI is the kid who invents crazy new cookie recipes.
- The predictive model is the parent who says: “No, you can’t put rocks in cookies. Let’s keep the ones with chocolate chips.”
- Only the safe, tasty recipes get baked in the oven.
- If the cookies come out yummy, the kid learns which recipes to try next time.
This discovery loop turns millions of theoretical designs into just a few promising AI-Designed Antibiotics.
The Promise and Challenge of AI-Designed Antibiotics
AI-Designed Antibiotics could mark the start of a “second golden age” in antibiotic discovery. For the first time in decades, pharma has a method to create new drug classes rather than rediscover old ones.
But the challenges are real:
- Manufacturing barriers: many AI-generated molecules are difficult to synthesize.
- Safety and efficacy: predictive modeling helps, but full toxicology and clinical trials remain mandatory.
- Economic hurdles: antibiotics are often unprofitable compared to oncology or chronic disease drugs.
BioIntel AI Analysis (What It Means for Pharma & Biotech)
1. A New Model for Antibiotic R&D
- Traditional discovery = digging through millions of random molecules.
- AI discovery = designing molecules on purpose, targeted to the problem.
2. Pharma’s Dilemma
- Antibiotics are critical for health but unprofitable because they are used sparingly.
- Business models must evolve: public funding, government incentives, or subscription-based reimbursement.
3. Beyond Antibiotics
- The same AI methods can accelerate discovery of cancer drugs, antivirals, and rare disease therapies.
- This is where pharma may see stronger commercial returns.
4. Regulatory Readiness
- FDA and EMA must develop standards for AI-designed molecules.
- Early engagement will give pharma leaders a say in how those rules are written.
>> Read our post : FDA’s 2025 Draft Guidance on AI in Drug Development: What It Means for Biopharma
Conclusion
The MIT team’s work shows that AI can move beyond screening existing molecules to designing entirely new ones. That’s a profound shift — and potentially the dawn of a new antibiotic era.
For pharma leaders, the question is no longer whether AI can contribute to drug discovery. It’s how to integrate AI-driven pipelines, navigate regulatory uncertainty, and build business models that make these innovations sustainable.
The antibiotics for gonorrhoea and MRSA may still be years away from patient use. But they already prove a larger point: AI is becoming indispensable in the fight against antimicrobial resistance — and beyond.
📎 External Resources:
- BBC – AI designs antibiotics for gonorrhoea and MRSA superbugs
- Using generative AI, researchers design compounds that can kill drug-resistant bacteria
- WHO – Global Antimicrobial Resistance Report
📬 Join 100+ life sciences professionals getting monthly AI insights. No spam, just signal.
